Metal-Organic Frameworks (MOFs), also known as porous coordination polymers (PCPs), are organic-inorganic hybrid coordination networks with potential voids composed of metal ions bridged by organic ligands. MOFs have attracted enormous research interests in recent years due to the diverse array of metal centers and organic ligands that can be combined to precisely tune surface area, pore volume, pore size, and functionality of the resulting porous solids. MOF materials are thus considered useful in a wide range of applications including gas storage and separation, catalysis, electronics, sensors and medicine. Significant research efforts in the field of MOFs have been focused on crystal structure designs and optimization through pre- and post-synthetic ligand variation in order to improve stability and to fine-tune chemical functionality. Relatively less attention has been paid to controlling the growth of MOF crystals at the microscopic scale and to the resulting MOF nanostructures that may exhibit distinct properties and reactivities when compared to corresponding bulk materials. Furthermore, assembly of MOF nanocrystals into well-ordered super-structures can result in new physical properties without the need to alter chemical identities.
We have discovered new mechanisms of controlled growth of one-dimensional (1D) MOF nano- and super-structures under size-confinement and surface directing effects have been discovered. Through applying interfacial synthesis templated by track-etched polycarbonate (PCTE) membranes, congruent polycrystalline zeolitic imidazolate framework-8 (ZIF-8) solid nanorods and hollow nanotubes were found to form within 100 nm membrane pores, while single crystalline ZIF-8 nanowires grew inside 30 nm pores, all of which possess large aspect ratios up to 60 and show preferential crystal orientation with the {100} planes aligned parallel to the pore’s long axis. Our findings provide a generalizable methodology for controlling size, morphology, and lattice orientation of MOF nanomaterials.
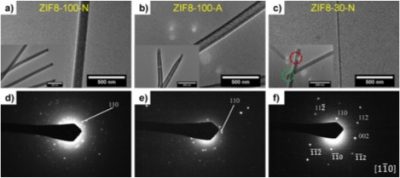
“Metal-Organic Framework (MOF) Nanorods, Nanotubes, and Nanowires.” Arbulu, R.; Jiang, Y.-B.; Peterson, E. and Qin Y.* Angew. Chem. Int. Ed. 2018, 57, 5813-5817. Link to Article
The majority of the MOF structures studied to date contain charge-neutral metal clusters as nodes, or secondary building units (SBUs), connected by organic struts that are also non-charged, leading to an overall charge-neutral framework. In these structures, the positive charges on metal atoms are immediately balanced by surrounding anionic ligands, e.g., carboxylates, and as a consequence, there are no exposed ionic species or accessible local electric fields within the MOF frameworks.On the other hand, a sub-class of MOFs known as ionic MOFs have emerged and attracted significant research attention. These ionic MOFs contain isolated charged centers, either positive or negative, at the metal nodes or on the organic ligands. The local electric fields, electrostatic interactions, and/or coordinating effects generated by these isolated charges can exert stronger interactions with polar substrates and molecules with high polarizability, than simple van der Waals interactions typically found in non-ionic MOF materials. Most of the ionic MOFs reported to date contain a single type of charge, either positive or negative, decorated covalently onto the framework, which leaves the free charge-balancing counter-ions within the pores. These counter-ions, depending on their sizes, unavoidably reduce accessible pore volumes of the MOF and can potentially occlude pore openings and inhibit adsorption of guest molecules. In this regard, it is appealing to incorporate both positive and negative charges at fixed distances and precisely controlled locations into one framework structure, forming the so-called charge-separated, or zwitterionic MOFs that can possess both the favorable interaction properties of ionic MOFs and free pore spaces as in non-ionic MOFs.
We have prepared a new charge-separated metal-organic framework (MOF), UNM-1 (C52H16BCuF16N4), possessing diamondoid structures, assembled from an anionic tetrahedral borate ligand and cationic Cu(I) metal ion. The resulting MOF structure displays four-fold interpenetration, resulting in high environmental stability, and at the same time possesses relatively large surface area (SABET= 621 m2/g) due to the absence of free ions. Gas adsorption measurements revealed temperature-dependent CO2 adsorption/desorption hysteresis and large CO2/N2 ideal selectivities up to ca. 99 at 313 K and 1 bar, suggesting potential applications of this type of charge-separated MOFs in flue gas treatment and CO2sequestration.
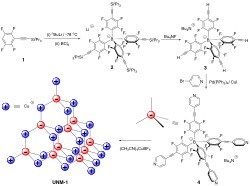
“A Charge-Separated Diamondoid Metal-Organic Framework.” Thapa, S.; Hettiarachchi, E.; Dickie, D. A.; Rubasinghege, G.* and Qin, Y.* Chem. Commun. 2018, Just Accepted. Link to Article